Bridging the gap in life science supply chains
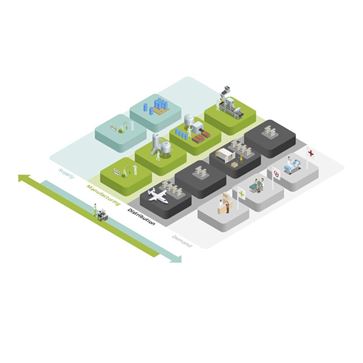
The life science supply chain is very dynamic with demand being fragmented and susceptible to unexpected shocks. Pharmaceutical companies are constantly engaged in making the right products in the right way at the right time, which requires a high level of organizational agility. Mission impossible? Unison Planning™ helps you manage all the internal and external operations, from chemical synthesis or biological fermentation through drug product manufacturing to the finished goods packaging, and distribution.
Dynamic environment
Life sciences supply chain planning is complex mainly due to fragmented demand across various sales channels. It is also highly susceptible to regulatory shocks, as well as disruptions from tenders and parallel trade.
These dynamics can make it a particular challenge for pharmaceutical companies to get all the right drugs to patients across the globe, appropriately dosed, packaged and labeled. Companies therefore have to be agile and flexible. They need to make careful adjustments to their distribution centers network, constantly monitoring finished goods and intermediate product inventory levels, while optimizing utilization of production and packaging lines.
Upstream production assets also need to be enhanced, taking into account quality and authorization constraints as well as CMO, dual-sourcing strategies and the financially-driven network strategy. Close collaboration and smart decision-making across the entire supply chain is crucial.
Breaking down barriers
Unison Planning™ makes all that happen. It brings all the complexities of the life science supply chain planning together in one model, including all the constraints and interdependencies of your assets and processes. This platform helps you manage all your activities in unison, breaking down the barriers between master production planning, detailed scheduling, demand planning, sales & operations planning, and strategic planning.
Clinical and commercial supply chains are managed as a whole, including interactions with suppliers, CMOs, and 4PL providers. As a result, reaction times are shorter, efficiency greatly improved, lead times shortened, safety stocks lowered and risks related to expiration or regulatory changes reduced. And you’ll successfully bridge the gaps between planning, quality assurance and control, finance, and the regulatory framework.
The results
Planners, managers and executives across your entire organization will be delighted. Full end-to-end visibility fosters collaboration throughout the supply chain. As a result, your operations are less fragmented and run smoothly.
Looking for real business results? Unlock enhanced visibility for your organization with Unison Planning™.
A tailored solution
What our customers say

“They truly are partners. They are very interactive and very proactive. At J&J we need that.”
Buz Hillman, Associate Director Clinical Supply Systems at Johnson & Johnson

“In the end, they delivered us a solution that we're very happy with. That delivered exactly what we needed.”
Dean Bergman, IT Director at Avanos Medical
“An optimal supply chain is critical to making sure people living with severe diseases obtain their medicines on time. OMP offered the quality and robustness in the planning process we were aiming for. Manual activities from multiple sources are now automated within Unison Planning, our new planning system of record. One common integrated process is now applied. This gives better control, greater visibility and a higher level of maturity, resulting in agile supply chains.”
Nathalie Loicq, Vice President Global Supply Chain at UCB

“Our selection happened in a very rational way and Unison Planning came out as the best choice.”
Arie Moruanx, Global Planning Excellence Leader – Medical Devices at Johnson & Johnson

“We were looking for an integrated, automated and scalable solution and that’s what we got from OMP.”
David Aherne, Senior Director Global Supply Chain at Alexion Pharmaceuticals